SUMMARY
The Ozone layer performs a critical role in the protection from ultraviolet radiation (UV). Although global ozone depletion has been ongoing, anthropogenic contributions of CFC and HFC emissions result in negative effects on human life. This paper explains what the ozone layer is, its protection of the earth, how it is impacted by man-made emissions, and what steps are being taken to mitigate its effect, including the International Day for the Preservation of the Ozone Layer.
INTRODUCTION
2.1 What is the Ozone Layer?
According to the National Geographic Encyclopedia entry, the ozone layer resides in the upper layer of the stratosphere and contains a concentration of ozone (O3) which absorbs 90 % of the ultraviolet light received by the earth. More than 90 percent of the ozone layers reside in the terrestrial atmosphere and are situated between 10 to 50 kilometers above the earth’s surface. This shield-like coating blocks solar radiation of wavelengths less than 290 nanometers(nm) and is the most influential feature of the earth that contains the earth’s atmosphere.
See Fig 1, depicting the layers of the atmosphere.
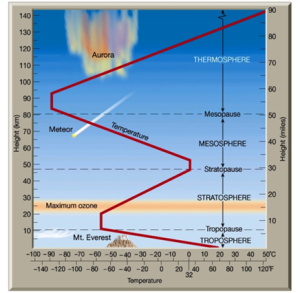
Fig 1. The layers of the atmosphere
2.2 The Ozone Layer Discovery
In 1913, famous French scientists, Henri Buisson and Charles Fabray discovered the ozone layer. It was then, that the two scientists measured the sun’s radiation emitted on the surface of the Earth as it is consistent with the spectrum of a black body that ranges in temperature between 5,500 to 6,600 K or (5000- 5700℃). Further research observed by the British Meteorologist G.M.B Dobson, the developer of the spectrophotometer, invented a device called the Dobson meter which measures the stratosphere where ultraviolet light and oxygen (O2) exist. While in today’s worldwide network the Dobson meter is used at ozone monitoring stations and the unit of measure is the Dobson unit which is equivalent to a layer of gas of a thickness of one-hundredth of a millimeter of the Earth. In 1957 the Dobson spectrophotometer managed by the British Antarctic Survey discovered a decline between the surface and space over the Halley Bay ground station during the spring. Theories of contributing chemicals that induce Antarctic depletion were presented to noble scientific bodies in 1995.
2.3 The Chemical reaction of the Ozone Layer
The chemical reaction of the ozone layer is incredibly unique. The photochemical process of photolysis explains how the ozone layer exists. The reaction occurs due to the ultraviolet light or visible light reacting with an oxygen molecule to break chemical bonds. The formation of the ozone layer in the stratosphere occurs from the Sun reactions with oxygen.
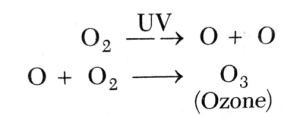
Figure 2. The molecular formation of ozone in the atmosphere
2.4 The Depletion of the Ozone Layer (Stratospheric Ozone)
Researchers Frank Sherwood and Mario Molina in the late 1970s gained awareness of specific chemicals that began influencing the ozone layer. The initiation of stratospheric ozone depletion influenced by anthropogenic activity (human activity) of pollutants containing gases such as bromine (Br) and chlorine (Cl) radicals is impactful on the depletion of the balance of the stratosphere. In further research by Frank Sherwood and Mario Molina, industrial chemicals such as carbon tetrachloride (CCl4) and methyl chloroform (CH 4CCl3) along with halons influenced the earth’s stratosphere. Furthermore, these halogen-derived gases are mostly reactive due to their Ozone Depletion Potential (ODP) estimated to be CFC-11 (stands for CC13F, trichlorofluoromethane), whereby gases with greater ODP damage the ozone layer over a period. The varying time can exist between 20-100 years. Below is a list of substances that impact the ozone layer.
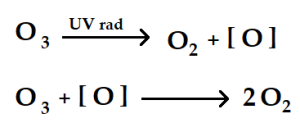
Figure 3. The molecular depletion of ozone layer
2.5 Causes of Ozone Layer Depletion
- Chlorofluorocarbons (CFC)- An organic substance composed of carbon, chlorine, and fluorine, manufactured as an unstable derivative of methane, ethane, and propane. It is a colorless, odorless, and chemically non-reactive gas that was originally designed to be used as a refrigerant in the 1900s by E. I du Pont de Nemours and Company. Some sources of CFCs are aircraft halons and aerosol sprays.
- Halons- Chemicals containing carbon, bromine, and fluorine destroy the ozone O3 molecule, eventually creating holes in the stratosphere’s ozone layer.
- Carbon tetrachloride- A dry cleaning solvent and ozone-depleting chemical with a lifespan of 20-100 years. When broken down into soil and water over a period it destroys the stratospheric layer.
- Hydrofluorocarbons (HFCs)- HFCs are industrial chemicals that form the primary component used for cooling and refrigeration. This powerful greenhouse gas is known to be a short-lived pollutant that has a lifespan of 14 and 29 years in the atmosphere. Although it represents 1% of greenhouse gases, the impact on global warming is much greater. The Kigali amendment under the Montreal Protocol phased out its use of it in 2019, with targets to void the metric tons of carbon equivalent emissions by the year 2050.

Figure 4. Depiction of the use of hydrofluorocarbon chemicals for refrigeration, air-conditioning, and aerosol propellants.
[Note. By HFC emissions sources and impact. Climate and Clean Air Coalition | ccacoalition.org]
In addition to the impactful effects of bromine and chlorine radicals, chlorine radicals participate in a catalytic cycle that destroys the ozone layer before it returns to the lower atmosphere. As a result, the Antarctic ozone layer also known as the “ozone hole” is a uniquely depleted section in the stratosphere over the Antarctic, beginning in the Southern Hemisphere.
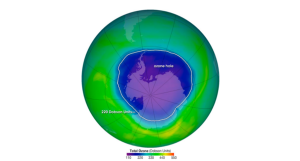
Figure 5. The Ozone over Antarctica with total ozone of 220 Dobson Unit
[Note. By NASA’s Aura Satellite. The ozone hole is the region over Antarctica with ozone of 220 Dobson Units or lower. This map shows the ozone hole on October 4, 2004.]
3 WORLD OZONE PRESERVATION
To highlight the need to protect the earth from harmful gases that threaten the ozone layer, Ozone preservation is celebrated on September 16th of every year since the UN General Assembly proclaimed it. The objective is to commemorate the signing in 1987, of the Montreal Protocol on substances that deplete the Ozone Layer and climate challenges. This then led to the drafting of the Montreal Protocol which encouraged countries to make efforts to reduce the use of harmful substances that may influence the ozone’s weakening. To date the United Nation Treaty Collection, there are forty-six signatories and 198 ratifiers.
Although various agreements are in the pipeline to curb ozone layer depletion such as the Paris agreement, other conventions were developed over time to preserve the ozone layer. The United Nations, Ozon Action described the Montreal Protocol as a multilateral environmental agreement aiming to control the production and utilization of artificialized chemicals known as ozone-depleting substances (ODS). Additionally, the Montreal Protocol seeks to triumph over climate change challenges influenced by ODS through global conventions. Under the treaty, all developed and developing countries are responsible to phase out the ODS in both the import and exportation of goods. The protocol further contains eight different articles to achieve the target and measurable commitments.
The Protocols include:
- Article 2- Control Measures
- Article 3- Calculation of control levels
- Article 4- Control of trade with non-parties
- Article 5 -Special situation of developing countries
- Article 7- Reporting of data
- Article 8-Non-Compliance
- Article 10- Technical assistance
Other Supporting Amendments
Vienna Convention– This environmental convention was developed in 1985 for the protection of the Ozone Layer, both Vienna Convention and Montreal Protocol became treaties to achieve universal ratification. They planned to provide mechanisms to deal with the challenges the ozone layer has been experiencing and put in place regulatory measures and frameworks.
Kigali Agreement- The Parties of the Montreal Protocol and multilateral agreements reduce ODS production and consumption by ensuring the agreement is monitored yearly. Through this, the Kigali Agreement on 15th October in Rwanda was developed to decrease the manufacturing of Hydrofluorocarbons (HFCs) by 85% and limit climate change.
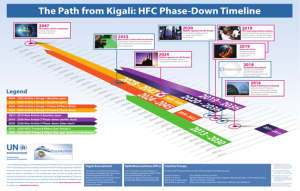
Figure 6. The Path from Kigali: HFC Phase-Down Timeline
[Note. By OzonAction. The Path from Kigali: HFC Phase-Down Timeline | Ozonaction (unep.org)]
4 EFFECTS OF THE OZONE LAYER DEPLETION
Studies have shown that the ozone layer shields ultraviolet radiation (UV) that causes sunburn. Ultraviolet radiation can hurt human health. UV radiation is classified into three types of high-level energy light which are often categorized, ultraviolet A, ultraviolet B, and ultraviolet C.
- UV-A
- 99 % of UV light reaches the earth’s surface in the form of UVA.
- Ages skin over a period.
- Increases the cause of melanoma and skin cancer.
- UV-B
- Small fraction reaches the earth’s surface and is highly influential to the ozone layer.
- Main cause of sunburn.
- Stimulates the production of vitamin D.
- UV-C
- Mostly absorbed by the components in the atmosphere such as nitrogen and oxygen, while the remaining rays are scattered.
- Kills microbes and bacteria on surfaces.
- Prolong exposure causes skin lesions.
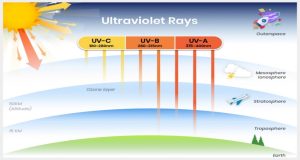
Figure 7. Ultraviolet Ray Spectrum and the Ozone Layers
The Ozone Layer Depletion Effects on Plants and Animals
Elevated levels of UV rays affect plant and animal life in many ways. Under extreme exposure to UV radiation, vegetation can become affected. The pores of the plant’s epidermis known as the stomata are burnt during the respiration process. Influential variables of air pollutants, soil moisture, and environmental stressors collectively enhance the degree of damage. In the case of animals, UV rays cause sun-induced damage to the epidermal layer of aquatic life. While terrestrial animals like sheep and cattle encounter diseases that cause blurry vision.
5 THE CURRENT STATE OF THE OZONE LAYER
Levels of ozone-depleting substances have increased before the application of the Montreal Protocol. Environmental specialists collectively argue that since the late eighty’s the health of the ozone layer is unfixed since the hole size differs by the existing temperature in the atmosphere. Although there are recovery mechanisms in place, recovery of the ozone layer is a complex accomplishment given the changes in the atmosphere fifty years ago (United Nations Environment Programme, n.d.). With the profound influence of global warming, greenhouse gases and global temperature impose on the environment, recovery may improve in some areas. However ongoing research is being conducted to underpin the link between the stratosphere, where the ozone layer resides, and the existing climate. Protecting the ozone layer and the life on Earth remains imperative to the well-being of agriculture, human well-being, and wildlife. Collaborative efforts for the success of the Montreal Protocol provide the globe with hope in tackling existing environmental challenges which include global warming, climate change, and sustainability.
CONCLUSION
The United Nations environmental programme (UNEP) and the joint efforts of other governments around the world cooperatively endeavor to reduce the depletion of the ozone layer with the 1987 Montreal Protocol for Ozone Depleting Substances. The gathered information highlights the concerns of researchers, regarding the acceleration of ozone and stratospheric layer depletion. Since greenhouse gases such as CFCs, Carbon tetrachloride, and HFCs are influential in the reduction of global warming, and climate change. The impact of ozone layer depletion is a threat to the quality of human, animal, and plant life and it requires ongoing vigilance to begin ozone recovery for future years.
References
- Canada, E. A. C. C. (n.d.). UV and the ozone layer – Canada.ca. Retrieved September 14, 2022, from https://www.canada.ca/en/environment-climate-change/services/weather-health/uv-index-sun-safety/ozone-layer.html
- Climate & Clean Air Coalition. Hydrofluorocarbons. (n.d.). Retrieved, September 15, 2022, from https://www.ccacoalition.org/en/slcps/hydrofluorocarbons-hfcs
- NASA Ozone Watch. (2018, October). What is the Ozone Hole? “NASA Ozone Watch.” https://ozonewatch.gsfc.nasa.gov/facts/hole_SH.html
- National Geographic Society. ozone layer | (n.d.). Retrieved September 14, 2022, from https://education.nationalgeographic.org/resource/ozone-layer/
- World Meteorological Organization (2021, September 15). Ozone layer recovery is an environmental success story. Retrieved September 14, 2022, from https://public.wmo.int/en/media/news/ozone-layer-recovery-environmental-success-story
- Sao, A. (2022, January). Ozone Layer Depletion – Causes and Effects: A Review. https://www.ijsr.net/archive/v11i1/SR22126125127.pdf.
- United Nations Environmental Programme.(2022). UNEP acting on the Montreal Protocol. https://www.unep.org/ozonaction/. Retrieved September 14, 2022, from https://www.unep.org/ozonaction/who-we-are/about-montreal-protocol
- UN Environmental Programme (2022).. 2022 World Ozone Day: Global Cooperation Protecting Life on Earth. https://www.unep.org/ozonaction/news/news/2022-world-ozone-day-global-cooperation-protecting-life-earth
- United Nations Environment Programme. (2022). While the ozone layer is healing, pitfalls remain. UNEP. Retrieved September 14, from https://www.unep.org/news-and-stories/story/while-ozone-layer-healing-pitfalls-remain
- UV Light. (2021, March 22). BYJUS. Retrieved September 14, 2022, from https://byjus.com/physics/uv-light/
